Henry’s Law
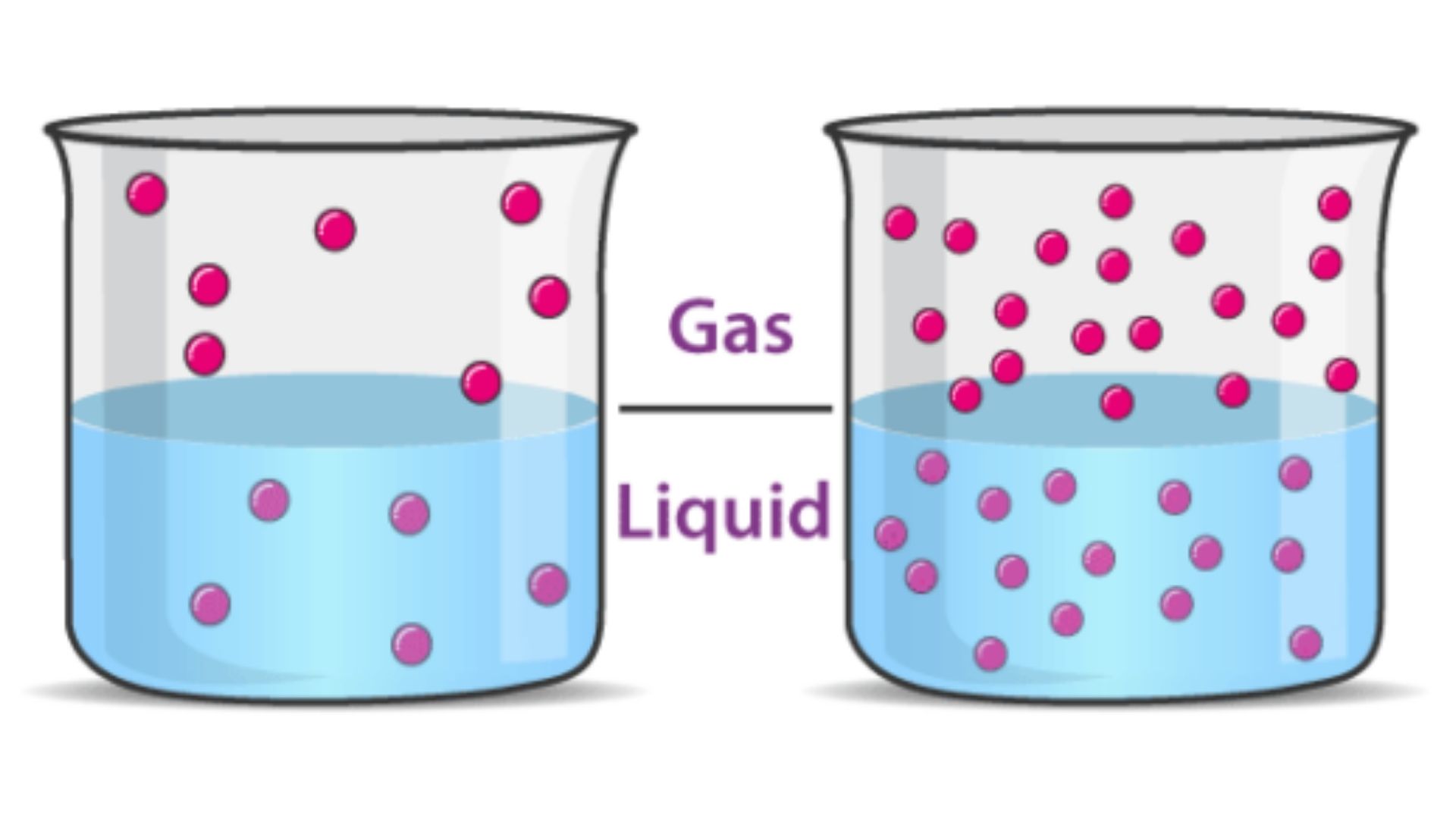
What is Henrys Law?
- A thermodynamic law that explains the dissoultion of a gas in a liquid medium.
At constant tempature, the amount of gas that will dissolve in a liquid is proportional to the partial pressure of that gas over the liquid.
As pressure is reduced, solubility decreases
- Most gas is excreted from the lungs
- Rapid ascend may lead to bubble formation
